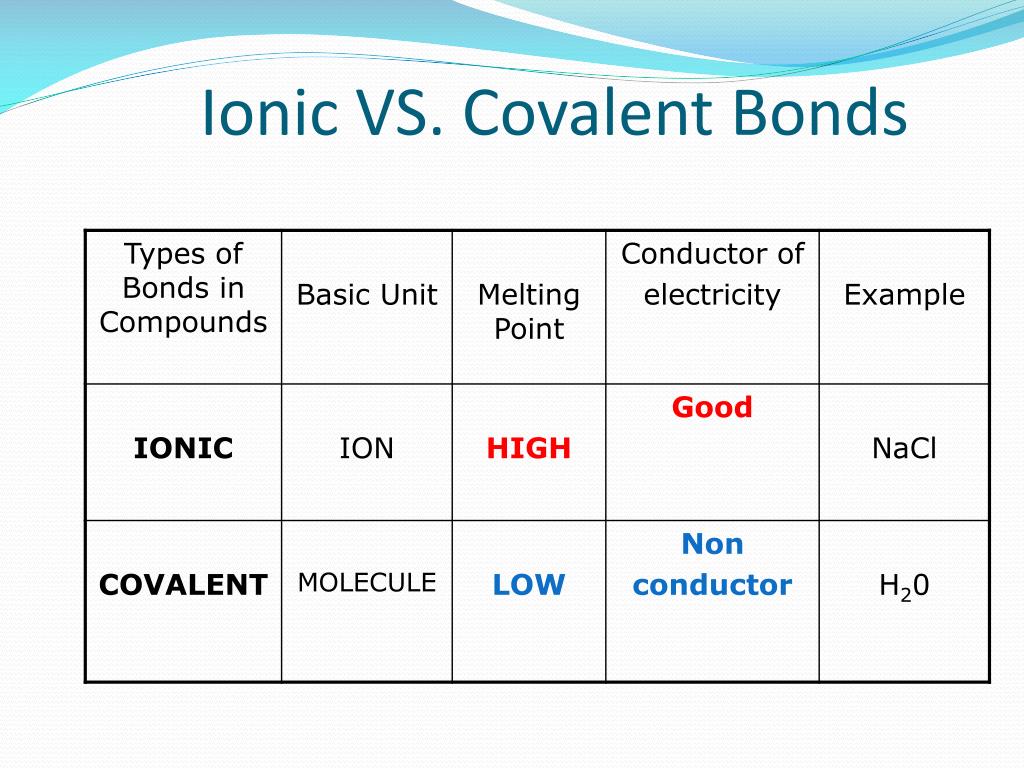
Ionic Bond Definition Vs Covalent . Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. Ionic bonds require at least one electron donor and one electron acceptor. Covalent bonds consist of pairs. Howstuffwoks explains ionic and covalent bonds. Here is an explanation of the. We’ll talk about what is an ionic bond, and what is a covalent bond. Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like ionic. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. They differ in their structure and properties. In ionic bonding, atoms transfer electrons to each other.
from www.slideserve.com
Howstuffwoks explains ionic and covalent bonds. We’ll talk about what is an ionic bond, and what is a covalent bond. Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like ionic. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Covalent bonds consist of pairs. They differ in their structure and properties. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one electron acceptor. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds.
PPT The Structure of Matter PowerPoint Presentation, free download
Ionic Bond Definition Vs Covalent Covalent bonds consist of pairs. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. In ionic bonding, atoms transfer electrons to each other. Howstuffwoks explains ionic and covalent bonds. Ionic bonds require at least one electron donor and one electron acceptor. Here is an explanation of the. They differ in their structure and properties. Covalent bonds consist of pairs. Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like ionic. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. We’ll talk about what is an ionic bond, and what is a covalent bond.
From www.slideserve.com
PPT Chemical Bonds (Ionic and Covalent Bonds) Part 1 PowerPoint Ionic Bond Definition Vs Covalent The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. We’ll talk about what is an ionic bond, and what is a covalent bond. Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like ionic. In. Ionic Bond Definition Vs Covalent.
From www.britannica.com
Covalent bond Definition, Properties, Examples, & Facts Britannica Ionic Bond Definition Vs Covalent Covalent bonds consist of pairs. Howstuffwoks explains ionic and covalent bonds. In ionic bonding, atoms transfer electrons to each other. We’ll talk about what is an ionic bond, and what is a covalent bond. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. Covalent bonding is a form of chemical bonding between. Ionic Bond Definition Vs Covalent.
From pediaa.com
Difference Between Covalent and Ionic Bonds Ionic Bond Definition Vs Covalent We’ll talk about what is an ionic bond, and what is a covalent bond. They differ in their structure and properties. Covalent bonds consist of pairs. Ionic bonds require at least one electron donor and one electron acceptor. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. Here is an explanation of. Ionic Bond Definition Vs Covalent.
From infogram.com
Covalent Bonds .VS. Ionic Bonds Infogram Ionic Bond Definition Vs Covalent Ionic bonds require at least one electron donor and one electron acceptor. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. They differ in their structure and properties. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Howstuffwoks explains ionic and. Ionic Bond Definition Vs Covalent.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Ionic Bond Definition Vs Covalent In ionic bonding, atoms transfer electrons to each other. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. Covalent bonds consist of pairs. They differ in their structure and properties. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of. Ionic Bond Definition Vs Covalent.
From ar.inspiredpencil.com
Covalent Bond Examples List Ionic Bond Definition Vs Covalent The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. We’ll talk about what is an ionic bond, and what is a covalent bond. Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like ionic. They. Ionic Bond Definition Vs Covalent.
From www.youtube.com
Properties of Ionic and Covalent Compounds YouTube Ionic Bond Definition Vs Covalent They differ in their structure and properties. Howstuffwoks explains ionic and covalent bonds. Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like ionic. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Here is. Ionic Bond Definition Vs Covalent.
From www.youtube.com
Ionic bond ,Covalent bond and Coordinate bond Chemistry Types of Ionic Bond Definition Vs Covalent Howstuffwoks explains ionic and covalent bonds. We’ll talk about what is an ionic bond, and what is a covalent bond. They differ in their structure and properties. Ionic bonds require at least one electron donor and one electron acceptor. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Covalent. Ionic Bond Definition Vs Covalent.
From techiescientist.com
Is SiO2 Ionic or Covalent? Techiescientist Ionic Bond Definition Vs Covalent Howstuffwoks explains ionic and covalent bonds. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. Covalent bonds consist of pairs. In ionic bonding,. Ionic Bond Definition Vs Covalent.
From www.teachoo.com
[Class 10] Differentiate between Ionic bond & Covalent bond Teachoo Ionic Bond Definition Vs Covalent In ionic bonding, atoms transfer electrons to each other. Howstuffwoks explains ionic and covalent bonds. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. The main difference between ionic and covalent bonds is how equally the electrons are shared between. Ionic Bond Definition Vs Covalent.
From www.currentschoolnews.com
Major Differences Between Ionic and Covalent Compounds People Ignore Ionic Bond Definition Vs Covalent Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like ionic. Covalent bonds consist of pairs. They differ in their structure and properties. Ionic bonds require at least one electron donor and one electron acceptor. Here is an explanation of the. In ionic bonding, atoms transfer. Ionic Bond Definition Vs Covalent.
From sciencenotes.org
Ionic Bond Definition and Examples Ionic Bond Definition Vs Covalent Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like ionic. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in. Ionic Bond Definition Vs Covalent.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Ionic Bond Definition Vs Covalent The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. In ionic bonding, atoms transfer electrons to each other. They differ in their structure and properties. Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like. Ionic Bond Definition Vs Covalent.
From sciencenotes.org
Ionic vs Covalent Bonds Ionic Bond Definition Vs Covalent They differ in their structure and properties. Howstuffwoks explains ionic and covalent bonds. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. In ionic bonding, atoms transfer electrons to each other. Covalent bonds consist of pairs. The main difference between ionic and covalent bonds is how equally the electrons are shared between. Ionic Bond Definition Vs Covalent.
From www.slideserve.com
PPT The Structure of Matter PowerPoint Presentation, free download Ionic Bond Definition Vs Covalent Ionic bonds require at least one electron donor and one electron acceptor. Here is an explanation of the. Howstuffwoks explains ionic and covalent bonds. Covalent bonds consist of pairs. They differ in their structure and properties. We’ll talk about what is an ionic bond, and what is a covalent bond. In ionic bonding, atoms transfer electrons to each other. In. Ionic Bond Definition Vs Covalent.
From chemistrylearnwithsangam.blogspot.com
chemistry knowledge Comparison between Covalent and Ionic Bond Ionic Bond Definition Vs Covalent Howstuffwoks explains ionic and covalent bonds. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. In ionic bonding, atoms transfer electrons to each other. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. We’ll talk about what is an ionic bond,. Ionic Bond Definition Vs Covalent.
From www.myxxgirl.com
Ionic Bond And Covalent Bond Venn Diagram Amashusho Images My XXX Hot Ionic Bond Definition Vs Covalent We’ll talk about what is an ionic bond, and what is a covalent bond. Here is an explanation of the. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. Covalent compounds usually have a lower boiling and melting point due. Ionic Bond Definition Vs Covalent.
From giovannigokesalas.blogspot.com
Difference Between Ionic and Covalent Bonds Ionic Bond Definition Vs Covalent They differ in their structure and properties. Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like ionic. In this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. Ionic bonds require at least one electron donor and one electron. Ionic Bond Definition Vs Covalent.